Veterinary Cardiologist Dr. Renee Riepe, DVM, DACVIM, shares an in-depth case study demonstrating how a positive, sustainable outcome can be achieved for a dog whose life expectancy was thought to be poor.
The Story of Loki's Heart
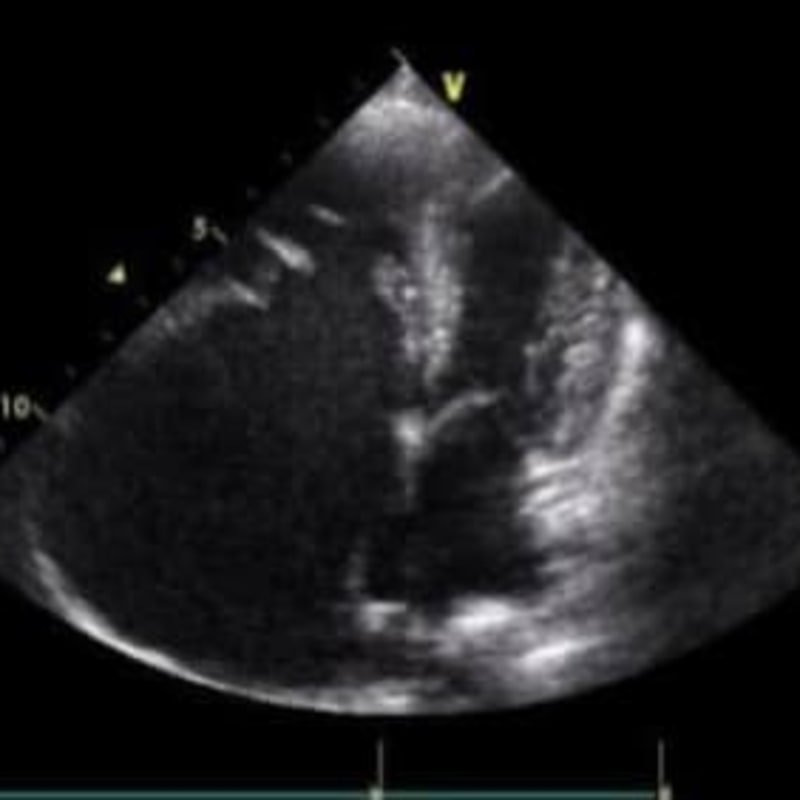
Tricuspid Valve Dysplasia (TVD) refers to malformation of any component of the tricuspid apparatus (annulus, chordae, leaflets, and/or the papillary muscles). In Loki’s case, his annulus (the ring of tissue the valve leaflets attach to) is much lower than normal and is displaced towards the right ventricle. His valve leaflets, chordae and papillary muscles were all malformed.
In these pictures, you can see how the right atrium dwarfs the size of the left atrium, because he had a very large volume of tricuspid regurgitation. You can also see how much lower the tricuspid valve annulus sits compared to the mitral annulus in the second picture (right heart on the left in this view).
Treatment Begins
When I saw him for the first time, I was hopeful that we might get a few happy years with him. He was initially started on Pimobendan, Furosemide, Spironolactone and Enalapril. While we typically do not start furosemide until a patient is in congestive heart failure, Loki’s heart enlargement was so extreme at such a young age that I decided it was best to dive in with a full congestive failure treatment plan.
I instituted the Pimobendan to enhance function and increase his cardiac output. Furosemide was used to reduce RV volume overload. Enalapril and spironolactone were added to block neurohormones that may contribute to fluid retention and myocardial fibrosis.
He did well until the following year when he presented in atrial fibrillation. The atrial fibrillation was a consequence of his marked right atrial enlargement. Fortunately, he did not go into congestive heart failure (ascites or pleural effusion), because when a patient goes into atrial fibrillation, the atria no longer contract and we lose 25% of forward blood flow. Because of the atrial fibrillation, the volume of blood in the right atrium increased and his risks for right congestive heart failure escalated.
When a dog with severe atrial enlargement goes into atrial fibrillation, we typically cannot convert the rhythm to normal because of the pathology of the severely dilated atrial myocardium. Because of this, we are typically left with medical management to slow the heart rate. Many patients in atrial fibrillation present with heart rates around 240 bpm. Fortunately, Loki’s atrial fibrillation rate was slow (100-140 bpm), so we did not have to add medications for heart rate control, but I did increase the pimobendan dose frequency to (mg/kg) three times/day to try to further maximize cardiac output.
Challenges in Treatment Arise
Loki did great, until 2022, when he was 7 years old. When he presented for his recheck, he had developed some ascites. Based on his kidney panel, I believed that he was developing furosemide resistance. I get a hint that this is happening when I keep increasing the furosemide dose, with no subsequent increase in the BUN to suggest that we are successfully diuresing the patient. In fact, if the BUN decreases, despite using a higher dose of furosemide, that is one sign that we are getting into trouble.
Diuretic resistance is a complex phenomenon that is not completely understood. One thought is that it is because of decreased kidney filtration and a delayed peak concentration of the loop diuretic (furosemide/torsemide) within the renal tubules. Another issue is hypertrophy of the cells within the distal renal tubule, which contributes to an increased reabsorption of sodium into the bloodstream. Third is that when cardiac output is poor, absorption of medications from the GI tract and subsequent delivery to the kidneys is delayed.
In people, NSAIDs can contribute to diuretic resistance. While we don’t focus on this in veterinary cardiology, it may be that it is being overlooked in our patient population.
Various approaches are taken to try to lessen the effects of diuretic resistance and patients frequently decompensating. In people, monitoring urine sodium loss and the ratio of urine creatinine to plasma creatinine may be helpful for detecting diuretic resistance. In veterinary medicine, because of costs, we cannot always do this type of intense monitoring, but we can recognize other clues that diuretic resistance is an issue.
One aspect is if I have a patient is decompensating frequently and the BUN is not increasing despite progressive furosemide dose increases, I will ask the owner, “Compared to when we first started furosemide, how frequently is he urinating now? How much does he urinate? Is it more or less than when we first started treatment? Invariably, the reply is, “Well, now that you mention it, he really doesn’t ask to go out as much as he did when he first started treatment.” We replaced furosemide with torsemide. In some studies, torsemide may have a greater bioavailability compared to furosemide. Torsemide is 10x more potent that furosemide.
Development of Pleural Effusion
He did great for another year, then developed pleural effusion. He had a thoracocentesis and around 2,400 mLs of fluid was drained from the pleural space. Based on his blood work, I started to have growing concerns that he was now becoming resistant to Torsemide as well as Furosemide. Now it was time for the dreaded “stacking” of diuretics.
Typically, when I add a thiazide to the treatment plan with Furosemide or Torsemide, it works really really well for stabilizing the congestive heart failure, but the electrolytes often drop and the BUN/Creat often skyrocket! The first step was to decrease the dose of the loop diuretic (Torsemide), then add in a low dose of the Hydrochorlothiazide. When this decision was made, he had been taking 20 mg of torsemide twice/day. Because Torsemide is 10x more potent than furosemide, this was effectively the dose equivalent of 200 mg of Lasix twice/day.
Before adding the thiazide:
- BUN = 35 ( 7-27)
- Creat = 1.3 ( 0.5-1.8)
- Potassium = 3.3 ( 3.5-5.8)
I decreased his Torsemide to 20 mg in the AM and 15 mg in the pm and added 25 mg of Hydrocholorothiazide in the AM and 12.5 mg at night. He was also taking Pimobendan, Spironolactone and Enalapril, but the focus of this story will be on the Torsemide and Thiazide combo.
After reducing the Torsemide and adding the Thiazide, his kidney panel looked like this one week later:
- BUN = 99 ( 7-27)
- Creatinine = 4.0
- Potassium = 2.8 ( 3.5-5.8)
He was no longer in congestive heart failure because this drug combination works, but as predicated, his BUN/Creat soared upward and his potassium decreased! The goal is to try to balance the positive and negative effects of this combination of diuretics.
Still Doing Well
To date, he has not gone back into congestive heart failure! After working with his treatment plan, as of August 2024, his torsemide dose is now only 2.5 mg twice/day (before adding the hydrochlorothiazide it was 20 mg twice/day) and his thiazide dose is 18.75 mg AM and 12.5 mg PM. This is a big difference from where we started with his torsemide dose.
In August of 2024, he weighed 35.06 kg and the other medications in the treatment plan includes the following:
- Pimobendan: 10 mg three times/day
- Spironolactone: 75 mg three times/day
- Potassium Gluconate: 550 mg tablet (2 tablets three times/day)
- Blood pressure via doppler was 110 mmHg.
- HR = 80-120 bpm (atrial fibrillation) No medications required for his heart rate control.
He is no longer on an ACE Inhibitor (enalapril) because of issues with his blood pressure. He was more vulnerable for hypotension, because the majority of his blood volume stays within his right heart and the atrial fibrillation decreases cardiac output by 25%.
What This Case Study Can Teach Us
The purpose of this case study is to show that with thought, effort, and an understanding of how to use the medications, we can have a positive, sustainable outcome for a patient whose life expectancy was thought to be poor. Also, I wanted to bring to light the topic of Lasix resistance and emphasize to NOT PANIC if the kidney values increase sometimes, because we can usually fix that as well as effectively treating the cardiac condition!
His kidney panel in August of 2024:
- BUN = 47 mg/dL (7-27)
- Creatinine= 2.4 mg/dL (0.5-1.8)
- Potassium = 3.7 ( 3.5-5.8)
What This Case Study Can Teach Us
The purpose of this case study is to show that with thought, effort, and an understanding of how to use the medications, we can have a positive, sustainable outcome for a patient whose life expectancy was thought to be poor.
Also, I wanted to bring to light the topic of Lasix resistance and emphasize to NOT PANIC if the kidney values increase sometimes, because we can usually fix that as well as effectively treating the cardiac condition!
His kidney panel in August of 2024:
- BUN = 47 mg/dL (7-27)
- Creatinine= 2.4 mg/dL (0.5-1.8)
- Potassium = 3.7 ( 3.5-5.8)
Loki in August of 2024, 9 years after his first appointment with me!